44 auxiliary labels drug reference chart
PDF Chapter 20 Labeling Medications and Expiration Dating and location, directions for use, and auxiliary labels c. Other labeling considerations: 1) The initials of the person preparing and verifying each compound 2) Placement of labels a) Affixed to containers so that they may be read while hanging b) Avoid covering manufacturer labeling containing drug name, Shop by Category | eBay Shop by department, purchase cars, fashion apparel, collectibles, sporting goods, cameras, baby items, and everything else on eBay, the world's online marketplace
Pharmacy Tech. : Auxiliary Label Chart Flashcards | Quizlet Pharmacy Tech. : Auxiliary Label Chart STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by mattvah Terms in this set (43) ACE Inhibitor -May Cause Dizziness -Avoid Alcohol -Take on empty stomach ADHD -Avoid caffeine -May cause insomnia -May be habit-forming Analgesic -May cause dizziness -May cause drowsiness Antibiotic

Auxiliary labels drug reference chart
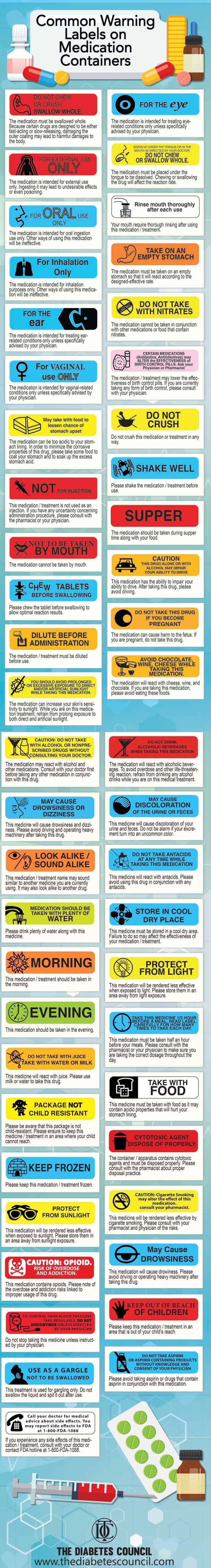
50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning. General Chapter Prescription Container Labeling - USP-NF Estimated proposal PF: 46 (1) Background and objective: General Chapter <17> Prescription Container Labeling provides universal standards for the format, appearance, content, and language of prescription medication instructions to promote patient understanding and reduce medication errors. The Healthcare Quality and Safety Expert Committee ... Guidelines for Prescription Labeling - The American Foundation for the ... Therefore, the Advisory Board recommends that pharmacies: Provide "duplicate labels" (prescription and auxiliary) printed in a minimum of 18-point type on paper stock. If pictograms are used, these should also be provided in "large print" format and high contrast (saturated black on white background).
Auxiliary labels drug reference chart. PDF Chapter 24 Medication Administration (Charting, Documentation and The ... When a PRN medication is administered, the nurse should properly document in the chart the following: a. The complaint or the symptom for which the drug was given. b. The dose, time, route of administration, and if appropriate the site of the injection. c. The results achieved or the statement no results achieved . d. The nurse's signature. PDF Pharmaceutical Compounding and Dispensing - Ducopharm important additional auxiliary labels. The summary list given in Table 1.1 is to be used as a guide in the absence of any guidance from the offi cial pharmaceutical texts. It should be remembered that the information in this table is to be used only as a guide. Any information on additional labelling or expiry dates in the offi cial texts PDF COMPLIANCE LABEL AND DRUG REFERENCE CHART • Ri 361 Steelcase Road West ... COMPLIANCE LABEL AND DRUG REFERENCE CHART • Ri 361 Steelcase Road West, unit Markham, Ontario L3R 3V8 Toronto 905-47 S -2500 Ton Free: :RTOIRE PDF NONSTERILE COMPOUNDING: BEYOND USE DATES and LABELING BEYOND USE DATES ... shortened in order to fit on the label. In the example above, Lid/Mlx/dip may not be used. If necessary, an auxiliary label stating complete drug names and amounts may be attached to the dispensing container in addition to the standard label. USP Chapter 795 requires the BUD and storage and handling information to be on the label.
PDF NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare ... drugs covered by the Alert were designed as thera - peutic agents for humans, human toxicity profiles should be given more weight than data from ani-mal models or in vitro systems. Additional guid-ance for defining hazardous drugs is available from the following sources: carcinogenicity [61 Fed Register 17960-18011 (1996b); IARC 2014], tera- Code of Laws - Title 40 - Chapter 43 - South Carolina ... SECTION 40-43-10. Short title; purpose of chapter; severability. This chapter may be cited as the "South Carolina Pharmacy Practice Act". The purpose of this chapter is to promote, preserve, and protect the public health, safety, and welfare by and through the effective control and regulation of the practice of pharmacy; the licensure of pharmacists; the licensure, permitting, control, and ... PDF Auxiliary Label Guiding Principles - BC Cancer developed the basic guiding principles for assigning auxiliary labels as follows: a. Auxiliary label information enhances but does not replace patient handouts or verbal counselling. b. A maximum of four auxiliary labels will be used due to container size limitation and to avoid alert fatigue. i. Exceptions: additional labels may be affixed to ... Auxiliary Medicinal Products in Clinical Trials information on the label shall be determined by the Member State concerned. The medicinal product may be labelled in several languages. 3.4 Safety reporting requirements for AxMPs This section applies to safety reporting requirement of adverse events suspected to be related to the AxMP only (= adverse reaction to AxMP). In case a suspicion of (or
Labels and Tape | United States | Nev's Ink Nev's Ink provides companies with high-quality labeling and tape solutions tailored to fit all of your business’s needs. Our state-of-the-art printing facility allows us to provide high quality, cost-effective solutions with some of the fastest turn times in the industry. Guidance Document: Labelling of Pharmaceutical Drugs for Human Use 3.8 Labelling of Professional Samples 3.9 Including International Information on Drug Package Labels Claims and Text Content 4.1 Misrepresentation of Classification 4.2 Absence of Ingredients 4.2.1 Sugar-free, Sucrose-free, Sweetener-free 4.2.2 Salt and Sodium-free 4.3 Absence of Side Effects 4.4 Side Effects and Placebo Comparisons Auxiliary Labels | Nev's Ink Auxiliary Labels Nev's Ink provides a wide variety of auxiliary labels that command attention and help you communicate with your staff. Our auxiliary labels are brightly colored and use easy-to-read and bold font so there is no chance of miscommunication. Healthcare Auxiliary Labels Veterinary Auxiliary Labels Guidance Document : Post-Notice of Compliance (NOC) Changes ... a sample of the inner and outer labels (Level I changes require label mock-ups while Level II changes require written text in place of mock-ups) to reflect any proposed changes. (d) An annotated and non-annotated electronic copy of the relevant Health Canada Quality Overall Summary template (QOS), or the revised sections of the QOS.
Auxiliary labels - Student Doctor Network DDS Applicants How To Admissions Guides Dental Admissions Guide Occupational Therapy Admissions Guide How to Get Into ... Can someone assist in locating list of auxiliary labels (e.g. refrigerate, shake, take with food, etc.) that correspond to a specific drug? ... In my pharmacy practice lab there was a printout that had a list of drugs, and ...
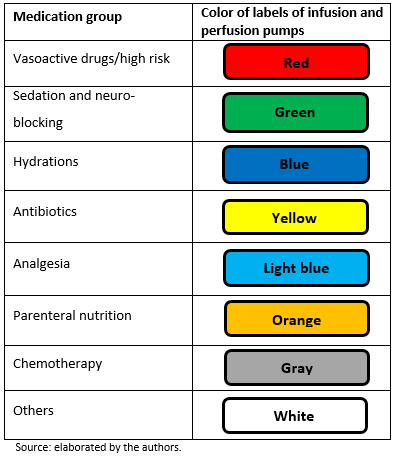
Predesigned labels to prevent medication errors in hospitalized patients: a quasi-experimental ...
Medication Guides | FDA Medication Guides are found within drug labeling - the section is usually located at the end of the drug label although this is not always the case. Note that the links in the Medication Guides...
Simple Strategies to Avoid Medication Errors - AAFP Home The Institute of Medicine (IOM) report Preventing Medication Errors estimated that 1.5 million preventable adverse drug events occur each year in the United States. 1 Another study estimated that ...
Safer dispensing labels for prescription medicines There is an emerging consensus for communicating less confusing, more informative and safer information on dispensing labels. 7-9 These labels have been called patient-centred labels, and recommendations have been developed based on research in health literacy and health communication (see Box 1). 3,10,11 Advice includes: use larger font sizes (e.g. 12 point and above)
PDF Revisions to APF24 Cautionary advisory labels Consult 'Drug interactions', page 111, a medicines information reference or the approved Product Information for more details. Label 12 revised explanatory notes Label 12 is recommended for medicines with the potential to cause CNS disturbances (e.g. dizziness, light-headedness, fatigue) and impair psychomotor performance.
PDF AUXILIARY LABEL - BC Cancer AUXILIARY LABEL . DATE: 1 June 2022. Page 1 of 14 . DRUG LABEL LABEL LABEL LABEL abemaciclib . abiraterone . acalabrutinib . acitretin . AFAtinib . alectinib . alitretinoin . anagrelide . AUXILIARY LABEL . DATE: 1 June 2022 Page 2 of 14 DRUG LABEL LABEL LABEL LABEL anastrozole . apalutamide .

A Comprehensive Approach to USP Compliance : November 2017 - Pharmacy Purchasing & Products Magazine
Auxiliary Labels | Nev's Ink AUXILIARY LABEL, REFILLED BY AUTHORITY OF YOUR DOCTOR - PAPER - PERMANENT - 3/8" X 1-1/2" - WHITE W/BLACK - 1,000 - ROLL PAUXW-0011 $8.18 AUXILIARY LABEL, GENERIC DRUG HAS BEEN DISPENSED AT LOWER PR - PAPER - PERMANENT - 3/8" X 1-1/2" - RED W/BLACK - 1,000 - ROLL PAUXW-0012 $8.18
PDF Lipitor (atorvastatin calcium) Label - Food and Drug Administration Drug therapy is recommended as an adjunct to diet when the r esponse to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. In pateinst wtih CHD or mutlpi el rsikfactors for CHD L, IPITOR can be started smi utlaneousyl wtih deit.
Preventing Medical Errors | Nursing CEU - CEUfast Place the medication list in a highly visible location in the patient's chart and including dosage, drug schedules, immunizations, and allergies or drug intolerances on the list. Create a process for reconciling medications at all care interfaces (admission, transfer, discharge) and determining reasonable time frames for reconciling medications.
Use of Booklet Labels on Investigational Medicinal Products (IMPs) - ISPE The objective of this concept paper is to reflect on a study site survey performed, to draw conclusions from an assessment on comparison of Booklet Labels versus Single Panel Labels performed, to discuss benefits of a Good Practice Guide, and to define the need for training on the proper use of booklet labels. The Good Practice Guide for ...
Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ...
PDF BuSpar - Food and Drug Administration any drug's use can be identified only after several years of marketing. Information for Patients To assure safe and effective use of BuSpar, the following information and instructions should be given to patients: 1. Inform your physician about any medications, prescription or non-prescription, alcohol, or drugs that
PDF Red C stamp CHAPTER Labeling thePrescription - LWW Using the labels created from the corrected prescriptions in Chapter 2, begin the process of filling and labeling prescriptions. Steps: 1. Separate the paperwork according to patient. 2. Begin filling the prescriptions for one patient. 3. Double-check each label with the hard copy of the prescription. 4.
PDF Good Label and Package Practices Guide for Prescription Drugs Good Label and Package Practices Guide for Prescription Drugs 9 3 Designing Labels and Packages for Safety 3.1 Introduction Part 3 of this guide presents information on current good practices in the design and layout of a health product label, the information contained on the label, and the design or choice of package.







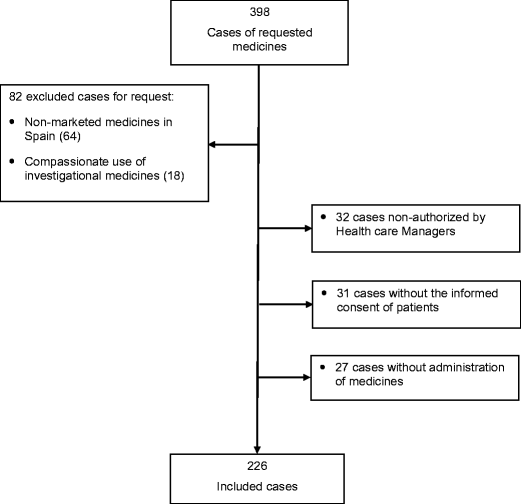

Post a Comment for "44 auxiliary labels drug reference chart"